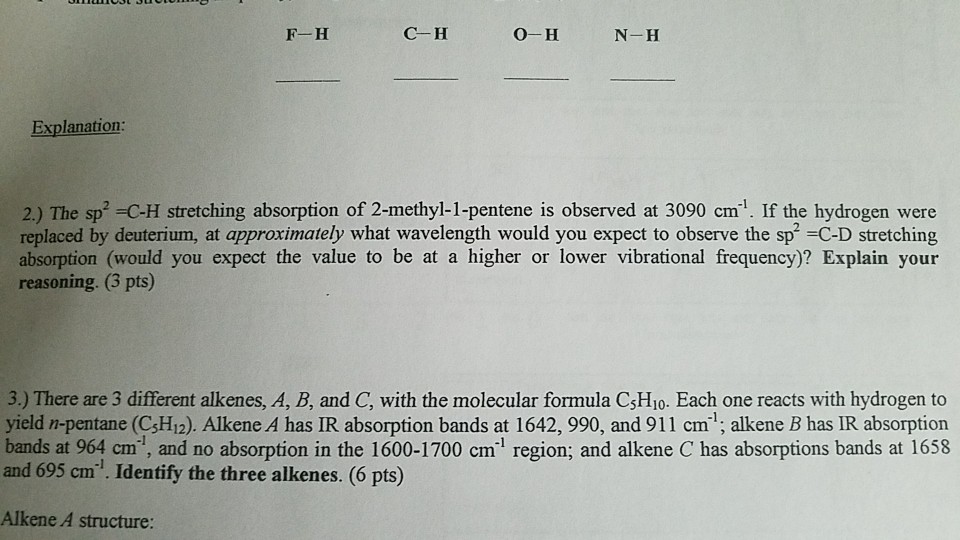
Show transcribed image text F-H C H O-H N-H Explanation 2.) The sp? -C-H stretching absorption of 2-methyl-1-pentene is observed at 3090 cm1. If the hydrogen were replaced by deuterium, at approximately what wavelength would you expect to observe the sp -C-D stretching absorption (would you expect the value to be at a higher or lower vibrational frequency)? Explain your reasoning. (3 pts) 3.) There are 3 different alkenes, A, B, and C, with the molecular formula C$H10. Each one reacts with hydrogen to yield n-pentane (CsHi2). Alkene A has IR absorption bands at 1642, 990, and 911 cm1; alkene B has IR absorption bands at 964 cm, and no absorption in the 1600-1700 cm1 region; and alkene C has absorptions bands at 1658 and 695 cm1. Identify the three alkenes. (6 pts) Alkene A structure:
F-H C H O-H N-H Explanation 2.) The sp? -C-H stretching absorption of 2-methyl-1-pentene is observed at 3090 cm1. If the hydrogen were replaced by deuterium, at approximately what wavelength would you expect to observe the sp -C-D stretching absorption (would you expect the value to be at a higher or lower vibrational frequency)? Explain your reasoning. (3 pts) 3.) There are 3 different alkenes, A, B, and C, with the molecular formula C$H10. Each one reacts with hydrogen to yield n-pentane (CsHi2). Alkene A has IR absorption bands at 1642, 990, and 911 cm1; alkene B has IR absorption bands at 964 cm, and no absorption in the 1600-1700 cm1 region; and alkene C has absorptions bands at 1658 and 695 cm1. Identify the three alkenes. (6 pts) Alkene A structure: